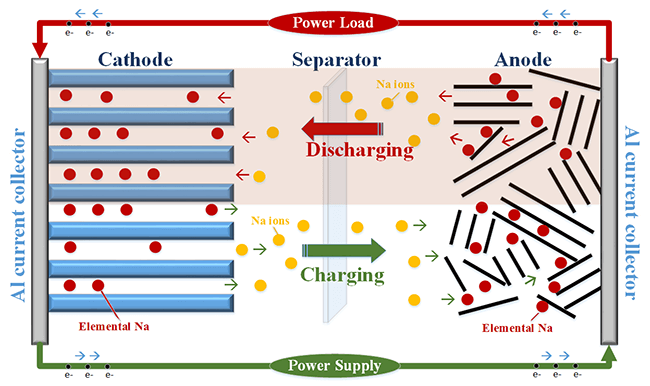
Sodium ion batteries work by storing and releasing energy. They use sodium ions to achieve this.
This type of battery offers an alternative to lithium-ion batteries, which are commonly used in electronics. Understanding sodium ion batteries is important as they promise cost-effective and sustainable energy solutions. As the demand for renewable energy grows, sodium ion batteries could become crucial.
Their working principle involves the movement of sodium ions between electrodes. This process enables energy storage and discharge. Research is ongoing to improve their efficiency and performance. Sodium ion batteries could be the answer to future energy needs, offering a greener option. Exploring their working principle helps grasp their potential impact on technology and the environment.

Credit: www.researchgate.net
Introduction To Sodium Ion Batteries
Sodium ion batteries are gaining attention in the energy storage world. They offer an alternative to traditional lithium ion batteries. As demand for sustainable energy solutions grows, sodium ion batteries emerge as a viable option. Their working principle is similar to lithium ion, but with sodium ions. This makes them an exciting field of study.
Emergence As An Alternative
Sodium ion batteries are being considered due to resource availability. Sodium is abundant and cost-effective compared to lithium. This makes production cheaper, benefiting industries and consumers. They provide a sustainable solution for large-scale energy storage. Their potential in grid storage and electric vehicles is being explored.
Comparison With Lithium Ion Batteries
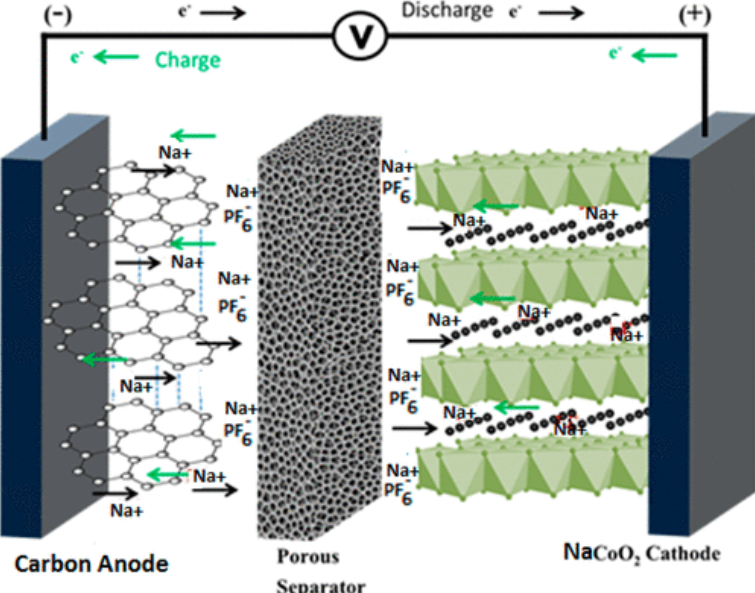
Both sodium and lithium ion batteries share similar structures. They both use a cathode, an anode, and an electrolyte. The main difference lies in the ion type. Sodium ions are larger than lithium ions. This affects energy density and efficiency. Sodium ion batteries generally have a lower energy density. Yet, they offer advantages in terms of cost and availability. Their environmental impact is potentially lower, making them eco-friendly.
Credit: www.ecolithiumbattery.com
Basic Structure Of Sodium Ion Batteries
Sodium Ion Batteries are gaining attention for energy storage. Their basic structure plays a crucial role in their efficiency. Understanding this structure helps us appreciate their potential.
Anode And Cathode Materials
The anode and cathode are the main components. Anodes usually contain hard carbon. This material allows sodium ions to move easily. Cathodes often use sodium-rich compounds. These include sodium cobalt oxide or sodium manganese oxide. These materials are vital for storing and releasing energy.
Electrolyte Composition
Electrolytes are essential in sodium ion batteries. They are usually liquid. They allow ions to travel between the anode and cathode. Sodium salts dissolved in solvents are common electrolytes. Proper composition ensures efficiency and stability. This makes the battery safe and effective for use.
Charge And Discharge Mechanism
Understanding the charge and discharge mechanism of a sodium-ion battery is crucial if you’re curious about its functionality. It’s the heart of how these batteries perform and store energy. This process involves the movement of ions and intricate electrochemical reactions, allowing the battery to release and absorb energy efficiently.
Ion Movement Process
The movement of ions is a dance of energy within the battery. During charging, sodium ions migrate from the cathode to the anode, creating a potential difference. This action is the battery’s way of storing energy for later use.
When you use your device, the ions journey back to the cathode. This reverse movement releases the stored energy, powering your device. It’s a fascinating cycle of movement and energy exchange.
Have you ever wondered why your device sometimes takes longer to charge? This is often due to the efficiency of ion movement. A smooth ion transition can mean faster charging times.
Electrochemical Reactions
At the core of the sodium-ion battery’s operation are the electrochemical reactions. These reactions occur at both the anode and cathode, facilitating the ion movement process. They’re like the behind-the-scenes workers ensuring everything runs smoothly.
During charging, the electrochemical reactions at the anode store the sodium ions. Conversely, during discharge, these reactions at the cathode release the ions, providing power. It’s a well-orchestrated process that needs balance.
Consider how you feel after a long day at work. Just as you need to recharge, the battery relies on these reactions to renew its energy. If the reactions are sluggish, the battery’s performance might lag.
Have you ever thought about how these mechanisms impact battery life? Efficient reactions can lead to longer-lasting batteries, making your device more reliable.
So, next time you’re waiting for your device to charge, think about the sodium ions bustling through your battery, ensuring you have the power you need. What questions do you have about the inner workings of your devices?
Role Of Sodium Ions
The role of sodium ions in sodium ion batteries is crucial. They are the primary charge carriers. Understanding their function helps us grasp how these batteries work. Sodium ions move between the battery’s electrodes during charging and discharging. This movement of ions is what powers the battery. Let’s explore their journey and impact.
Sodium Ion Transport
Sodium ions travel through the electrolyte. This is the battery’s liquid or solid medium. The journey begins at the anode. During charging, sodium ions leave the anode. They move through the electrolyte to the cathode. This process is called intercalation. During discharge, the ions return to the anode. This flow of ions creates electrical energy. That powers devices.
Impact On Battery Performance
Sodium ions affect the battery’s performance directly. Their size is larger than lithium ions. This impacts energy density. Sodium ion batteries usually have a lower energy density. But they are more abundant and less expensive. Their transport speed affects charging time. Faster ion movement means quicker charging. The efficiency of sodium ion transport influences battery life. Efficient ion movement extends the battery’s lifespan. Understanding these factors helps improve battery design.
Advantages Of Sodium Ion Technology
Sodium ion technology is gaining traction in the world of energy storage. The advantages it offers are compelling, especially when compared to traditional lithium-ion batteries. You’ll find that sodium ion batteries are not just about storing power; they’re about doing it efficiently and responsibly. Let’s dive into why they might be the right choice for your energy needs.
Cost Efficiency
One of the standout benefits of sodium ion batteries is their cost efficiency. Unlike lithium, sodium is abundant and cheaper to extract. This means lower production costs, which translate into savings for consumers. Imagine being able to power your devices or even your home without breaking the bank.
Think about how often you replace gadgets or upgrade your tech. With sodium ion batteries, the initial investment is lower, allowing for more frequent upgrades without hefty costs. This can change the way you approach technology purchases.
Environmental Benefits
Sodium ion batteries offer significant environmental advantages. The extraction of sodium is less damaging to the earth compared to lithium. This results in reduced environmental degradation, making sodium a more sustainable choice.
Consider the footprint you leave behind. Opting for sodium ion technology means contributing to a healthier planet. It’s a choice that aligns with the growing need for eco-friendly solutions. You could be part of a movement towards cleaner energy.
These batteries also boast better recyclability. Picture a future where battery waste is minimized, and recycling is maximized. Sodium ion batteries could pave the way for such a future, offering a tangible solution to a pressing problem.
Are you ready to embrace a technology that saves money and protects the planet? Sodium ion batteries might just be the answer you’ve been looking for. Their benefits are not only practical but also align with the values of sustainability and efficiency. It’s time to consider how these advantages could impact your life and the world around you.
Challenges Facing Sodium Ion Batteries
Sodium ion batteries operate by moving sodium ions between an anode and a cathode during charging and discharging. Challenges include lower energy density and shorter lifespan compared to lithium-ion batteries. These issues hinder their widespread use in high-demand applications.
Sodium-ion batteries are gaining attention as a potential alternative to lithium-ion batteries. However, they aren’t without their challenges. From energy density constraints to stability issues, these batteries must overcome significant hurdles before becoming a mainstream energy solution. Let’s dive into the primary challenges facing sodium-ion batteries.
Energy Density Limitations
Sodium-ion batteries often face limitations when it comes to energy density. Energy density refers to the amount of energy a battery can store relative to its weight. Unfortunately, sodium-ion batteries tend to lag behind their lithium counterparts in this area. This is primarily because sodium ions are larger and heavier than lithium ions. This size difference leads to less efficient packing and, consequently, a lower energy density. Imagine carrying around a heavier backpack that holds less than your usual one; that’s what sodium-ion batteries currently feel like. The challenge is significant: how do you make something heavier store more energy efficiently? Researchers are tirelessly working on this, but a breakthrough is still needed.
Material Stability Issues
Material stability is another significant concern for sodium-ion batteries. The materials used in these batteries can degrade over time, reducing their lifespan and reliability. Sodium is more reactive than lithium, which can lead to quicker wear and tear on the battery components. This reactivity can cause problems, such as the formation of dendrites. These needle-like structures can short-circuit the battery, posing safety risks. If you’ve ever had a phone battery die unexpectedly, you know how frustrating unreliable batteries can be. The challenge is to develop materials that can withstand this reactivity and maintain stability over many charge and discharge cycles. So, what does this mean for you? It suggests that while sodium-ion batteries hold promise, they need more refinement. Would you trust a battery that might not last as long or deliver as much power as you need? It’s a question both consumers and researchers are actively considering.
Recent Advances In Sodium Ion Battery Research
Sodium ion battery research is advancing rapidly. Researchers are exploring various areas to improve efficiency. These advancements aim to make sodium ion batteries viable alternatives to lithium-ion batteries. They focus on finding better materials and designs. This helps enhance performance and reduce costs.
Innovative Material Discoveries
Scientists are discovering new materials for sodium ion batteries. These materials improve capacity and stability. One example is the use of layered metal oxides. They offer high energy density. Another promising material is Prussian blue analogs. They provide better electrochemical properties. Carbon-based materials are also being explored. They enhance conductivity and storage capabilities.
Improved Battery Designs
Researchers are working on improved designs for sodium ion batteries. These designs aim to increase lifespan and efficiency. One approach is developing better electrode structures. These structures improve ion transfer rates. Another focus is on electrolyte optimization. It helps ensure safe and stable battery operation. Solid-state electrolytes are being investigated. They promise higher safety levels and longer battery life.
Credit: www.researchgate.net
Future Prospects Of Sodium Ion Batteries
Sodium ion batteries are gaining attention. They promise to be cheaper than lithium batteries. Abundant sodium resources make them appealing for sustainable energy solutions. Their potential benefits include better environmental impact and cost-effectiveness. Researchers are exploring ways to enhance their performance.
Potential Market Applications
Sodium ion batteries could power electric vehicles. Their lower cost makes them suitable for large-scale energy storage. They might be used in grid applications. This ensures stable energy supply from renewable sources. Portable electronics could benefit from these batteries. They offer a viable alternative where lithium is scarce.
Predicted Technological Developments
Advancements in electrode materials are expected. These developments could improve battery life and efficiency. Scientists are working on optimizing electrolytes. This could enhance charge and discharge rates. Future designs may focus on increasing energy density. Such improvements might make them competitive with lithium batteries.
Frequently Asked Questions
What Are The Downsides Of Sodium-ion Batteries?
Sodium-ion batteries have lower energy density compared to lithium-ion batteries. They are heavier and bulkier, affecting portability. Limited commercial availability and shorter lifespan are additional drawbacks. These factors make them less suitable for high-performance applications. Cost-effectiveness is still a challenge in scaling production for widespread use.
Why Are Sodium Batteries Not Used?
Sodium batteries aren’t widely used due to lower energy density compared to lithium-ion batteries. They also face challenges with efficiency and scalability. Sodium’s larger atomic size limits performance, making them less suitable for compact devices. Research is ongoing to improve these aspects and make sodium batteries more viable.
What Is The Lifespan Of A Sodium-ion Battery?
A sodium-ion battery typically has a lifespan of around 2,000 to 3,000 charge cycles. This depends on usage and conditions. These batteries offer a promising alternative to lithium-ion, with cost-effectiveness and sustainability. Regular maintenance and optimal usage conditions can help extend their lifespan.
How Soon Will Sodium-ion Batteries Be Available?
Sodium-ion batteries are expected to be commercially available by 2025. Companies are actively developing and testing prototypes. These batteries promise to be more cost-effective and sustainable than lithium-ion alternatives. Keep an eye on industry announcements for the latest updates on their release.
Conclusion
Sodium ion batteries offer a promising energy solution. They work by moving sodium ions. This movement happens between electrodes. It helps store and release energy. Sodium is abundant and cheaper than lithium. This makes these batteries appealing for future use.
Their environmental impact is also lower. Research continues to improve their efficiency. More advancements could lead to wider adoption. Understanding their principle aids in appreciating their potential. They might soon become a key player in energy storage. Keep an eye on this evolving technology.
It holds exciting possibilities for sustainable power.